Selected links to NICE guidance on atrial fibrillation, with a few notes and quotations.
- Consider referral for cardioversion for people whose symptoms continue after heart rate has been controlled or for whom a rate‑control strategy has not been successful.
- Refer people to be seen within four weeks if treatment at any stage fails to control the symptoms of AF and specialist input is required.
In this context, "cardioversion" may mean "pharmacological cardioversion", since it is contrasted with "rate control" - see notes on Cardioversion below.
Last revised in August 2022.
NICE BNF: Treatment summary: Atrial fibrillation
Refer to cardiologist if significant ECHO abnormalities.
"Prescribe beta blocker as first line ...Aim for heart rate < 80 bpm at rest; accept resting heart rate up to 110 bpm". I.e. prescribe rate-control drug, not rhythm control.
"Consider trial of sinus rhythm by cardioversion in 1st presentation of AF. Review stable patients every 6 months and after any change in treatment." It's possible that "cardioversion" here means "pharmacolgical cardioversion", i.e. the use of rhythm control drugs - see notes on Cardioversion below.
Refer to cardiologist if "Symptomatic (SOB, dizzy, tired, palpitations) despite strict rate control (resting HR < 80bpm & exercise HR <110bpm), vagal AF suspected, Arrhythmias - WPW Syndrome, tachy-brady syndrome, uncontrolled ventricular rate, Heart Failure, Arrhythmia post AF ablation". My bold - you have all three of these symptoms (I assume "SOB" is "shortness of breath").
Dated Jan 2018, due for review Jan 2021.
"NICE advises that all patient with paroxysmal AF should be referred to a cardiology specialist, but patients preferences should be taken into account", but includes pathways where initial treatment is handled in primary care.
Dated 2018.
Only refer to cardiologist if significant abnormalities in Echo or poor response to medication, so similar to South East.
Dated 2018.
"If patient remains symptomatic despite optimal rate control, or if a rhythm control strategy more appropriate – refer to Cardiology".
Refer to cardiologist "If more specialised treatment is needed e.g. new onset AF (<48 hours) for consideration of cardioversion; when a rhythm control strategy would be more suitable; those with atrial flutter who may be suitable for ablation; heart failure caused primarily by AF; age <65 years for assessment of treatment strategy; when valve disease is suspected; management of paroxysmal AF."
Dated 2019. Review date Nov 2022.
There seems to be a growing concensus that catheter ablation (CA) should be used as a "first-line" treatment for AF in preference to antiarrhythmic drugs (AADs). It seems that AADs are not very effective in preventing AF (maintaining sinus rhythm), and their adverse effects are as bad as those of AF (although different).
There are lots of papers and articles on the topic - some are listed below:
The NICE guidance on AF was last updated in 2021 and still recommends first-line use of AADs, and only recommends ablation if "drug treatment is unsuccessful, unsuitable or not tolerated in people with symptomatic paroxysmal or persistent atrial fibrillation". The rationale for this decision doesn't seem to consider first-line use of ablation, probably because most of the papers on first-line ablation were published too late to be included.
The evidence review for ablation only looked at studies published up to 2020. It included some early studies of first-line ablation (latest was 2017), but they seem to have been thrown into the mix ("pooled meta-analysis") with the other studies where the patients had already had AADs and possibly prior ablations. The quality of evidence was low or very low for all but two of the studies considered. There's an interesting quote in section 1.7.1.3 page 82 (my bold):
The committee agreed that medical treatment had the highest rate of recurrence but the lowest rate of stroke, and that the catheter ablation treatments appeared to have similar efficacy and harms to each other. The committee discussed the higher risk of stroke evident from the data for radiofrequency multielectrode (RF ME) treatment, whilst noting that some of the devices responsible for the higher risk had since been discontinued. Based on this pairwise evidence, the committee concluded that the different ablation techniques appeared to have comparable balances of benefits and harms for paroxysmal AF patients. Whilst ablation appeared to be clearly superior to medical care, both for first line patients and those who had failed at least one anti-arrhythmic drug, the committee recognised that comparisons between ablation techniques were made somewhat complex and unclear by the many pairwise comparisons made.
"There are two kinds of cardioversion. Your doctor may give you one or more medications to bring back your regular heartbeat. This is called pharmacologic (chemical) cardioversion. Doctors also restore regular rhythms by sending an electrical shock to the heart. This is called electrical cardioversion."
Confusingly, "cardioversion" is often used on its own to mean "electrical cardioversion", and, I suspect, sometimes to mean "pharmacological cardioversion".
2020 paper by a Danish group.
"Cardioversion, either by a synchronized direct current (DC) electrical shock (electrical cardioversion, ECV) or by the application of antiarrhythmic drugs (AADs; pharmacological cardioversion, PCV), is an integral part of the management of atrial fibrillation (AF) and atrial flutter (AFL) in symptomatic patients who require a rhythm control strategy."
"Electrical cardioversion terminates AF in over 90% of cases and
is the treatment of choice in severely haemodynamically compromised
patients with new-onset AF or AFL."
'new-onset' probably means what NICE call 'acute'.
"Complications of rhythm control with ECV include sedation-related complications, hypotension, ventricular fibrillation due to inappropriate shock synchronization, bradycardias (frequently diagnostic, i.e. unmasking sick sinus or sick atrioventricular node syndrome), tachycardias, such as AFL with 1:1 conduction or torsade de pointes." My bold.
"Complications of ECV and PCV are generally rare." But with ECV 1.1% had heart failure and 1.3% had major bleeding.
"In most patients with recent-onset AF, immediate cardioversion may be replaced by a wait-and-see approach as the default approach with delayed cardioversion as needed."
"recent-onset or paroxysmal AF may terminate spontaneously (Figure 3). Antiarrhythmic drugs foreshorten time to sinus rhythm but at the end of the day, numbers of patients in sinus rhythm are the same."
"Although cardioversion of AF or AFL is considered safe in general, cardioversion is associated with an increased risk of thromboembolic events." "Thromboemboli after cardioversion are considered due to embolization of already existing thrombi present in the atrium at the time of cardioversion or to the formation and subsequent embolization of de novo thrombi in the atrium that form while atrial function is still depressed in the weeks after cardioversion." Anticoagulants reduce the risk. "Almost all thromboembolic events with cardioversion occur within 10 days after the procedure. Therefore, anticoagulation up to 4 weeks after cardioversion is recommended."
"The peri-cardioversion rates of stroke, systemic embolism, and bleeding are low with NOACs."
Conclusion: "Cardioversion is widely used as part of a rhythm control strategy in patients with AF. Nevertheless, a wait-and-see approach is reasonable in patients with recent-onset AF, as the majority will convert spontaneously within 48 h." "Although complications of cardioversion overall are rare, it is of utmost importance to assess thromboembolic risk before the procedure, initiate timely OAC and continue life-long in patients with increased stroke risk. The advent of NOACs facilitates the streamlining of the peri-procedural anticoagulation management and, thus, performing cardioversion without major delays, provided that patients have been adequately counselled about the necessity for compliance to NOAC treatment." My bold.
"The AFFIRM study tried to determine whether electrical cardioversion and antiarrhythmic drugs to maintain sinus rhythm were better than drugs to slow atrioventricular node conduction, controlling ventricular response." "the AFFIRM study showed that management of atrial fibrillation based on control of heart rhythm did not offer any survival advantage compared to a strategy based on heart rate control."
"In the PIAF study ... Only 10% of patients with heart rate control had sinus rhythm after one year, compared to 50% of patients in the group treated with cardioversion, though patients in this group were hospitalized more often because of repeated cardioversion treatment."
"Recently, the therapeutic options for treating atrial fibrillation have been extended by the introduction of new cardioprotective agents which are able to prevent remodeling or ion channel modification." These include ACE inhibitors, which reduce blood pressure by relaxing blood vessels.
"Despite these pharmacological options, we should not forget that catheter ablation has been effective in the treatment of various types of arrhythmia." "We believe that the treatment strategies proposed in response to the results from the AFFIRM and RACE studies are not the best ones." "Patients with atrial fibrillation should also be pretreated before submitting them to electrical cardioversion. Such pretreatment is improving continually."
"The immediate reaction to the AFFIRM study is that we should forget cardioversion of atrial fibrillation, but we think that such a response is clearly wrong. Instead, new recommendations should be established". " Ablation may be the therapeutic option of choice in certain patients with symptomatic paroxysmal atrial fibrillation that recurs despite medical treatment."
"CONCLUSIONS: Older patients with persistent asymptomatic atrial fibrillation and risk factors for embolism are candidates for control of ventricular rate and chronic administration of anticoagulants. Electrical cardioversion is still clearly justified in many patients. Patients with episodes of recurring and refractory paroxysmal atrial fibrillation are ideal candidates for catheter ablation." My bold.
There are 10 standard electrode positions used in the 12-lead ECG. Table from Wikipedia:
| Electrode name | Electrode placement |
|---|---|
| RA | On the right arm, avoiding thick muscle. |
| LA | In the same location where RA was placed, but on the left arm. |
| RL | On the right leg, lower end of inner aspect of calf muscle. (Avoid bony prominences) |
| LL | In the same location where RL was placed, but on the left leg. |
| V1 | In the fourth intercostal space (between ribs 4 and 5) just to the right of the sternum (breastbone) |
| V2 | In the fourth intercostal space (between ribs 4 and 5) just to the left of the sternum. |
| V3 | Between leads V2 and V4. |
| V4 | In the fifth intercostal space (between ribs 5 and 6) in the mid-clavicular line. |
| V5 | Horizontally even with V4, in the left anterior axillary line. |
| V6 | Horizontally even with V4 and V5 in the mid-axillary line. |
The term "lead" actually refers to an electrode referenced to another electrode or the average of a group of electrodes. There is no 'common' electrode to which all other electrodes are referred. See Wikipedia.
Lead I is the voltage between the (positive) left arm (LA) electrode and right arm (RA) electrode.
Lead II is the voltage between the (positive) left leg (LL) electrode and the right arm (RA) electrode.
This article and guide on single-lead ECGs may be useful for interpretation. These are for hand-held devices (like Kardia) which produce an ECG waveform similar to lead I of a 12-lead ECG. The specification for the Wellue ECG includes "Lead set: lead II", which may mean it produces a waveform similar to lead II of a 12-lead ECG.

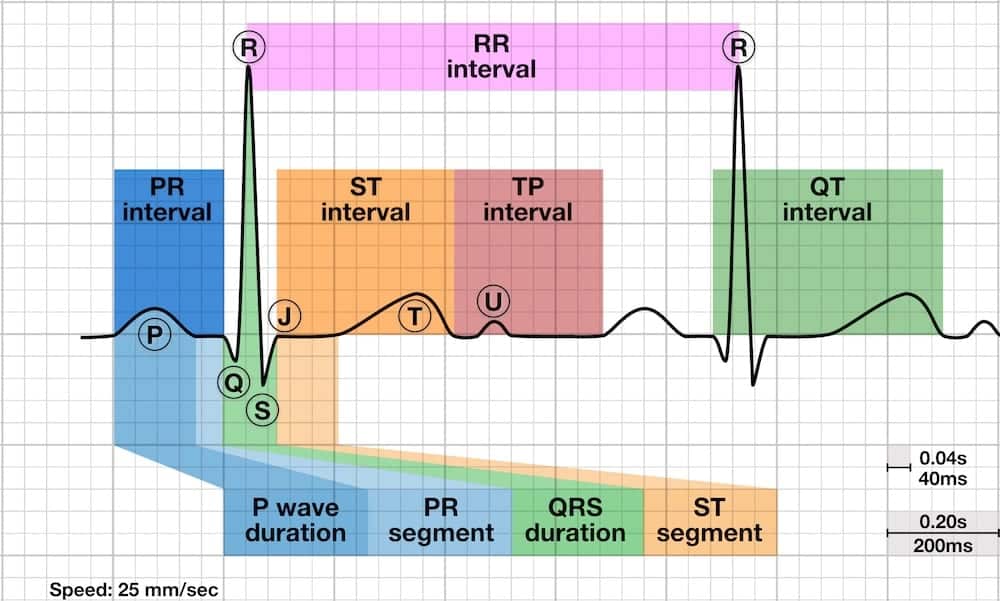
| Name | Normal range | If long | If short | Monica | Frank |
|---|---|---|---|---|---|
| RR interval | |||||
| PR/PQ interval | 120 – 200 ms (0.12-0.20s) in duration (three to five small squares) | First degree heart block. | Pre-excitation (the presence of an accessory pathway between the atria and ventricles) or AV nodal (junctional) rhythm. + delta-wave = Wolff-Parkinson-White syndrome (WPW), risk of a circus movement tachycardias (= AVRT: AV re-entry tachycardia). | 100-120mS | 100-120mS |
| ST interval | |||||
| TP interval | |||||
| QT interval | |||||
| P wave duration | < 0.12 s (< 120ms or 3 small squares) | > 110 ms with peak followed by trough: left atrial enlargement. Hyperkalemia. | |||
| Q wave duration | < 40 ms (1 mm) wide | Current or prior myocardial infarction | |||
| PR segment | |||||
| QRS duration | < 120 ms 60-100mS | Bundle branch block | 60-80mS | 320-360mS | |
| ST | |||||
| QTc | male < 460 ms, female < 450mS 390-450mS | Hypokalemia, postinfarction, long QT syndrome, medication. | Hypercalcemia. | 370-460mS | 410-510mS |
QTc is corrected QT interval - QT interval divided by square root of (RR divided by 1 second).
See CV Physiology: Non-Pacemaker Action Potentials, CV Physiology: Ion Channels, The Cardio Vascular System, Cardiac Ion channels
These are are characteristic of atrial and ventricular myocytes. The specialized conducting cells found within the His-Purkinje system of the ventricles are similar, except that they exhibit spontaneous depolarization.
| Phase | Description | Fast Na+ in | Transient K+ out | K+ out | Ca++ in | Potential mV |
|---|---|---|---|---|---|---|
| 0 | Rapid depolarization | C-O -60mV | C | C | C-O -40mV | -90 .. +30 |
| 1 | Brief repolarization | OI small | O | C | O | +30 .. 0 |
| 2 | Plateau | OI small | C | C | O | 0 .. -10 |
| 3 | Repolarization | I-C | C | O increased | I | -10 .. -90 |
| 4 | Resting state | C | C | O | C | -90 |
See CV Physiology: Sinoatrial Node Action Potentials
See Cardiac transmembrane ion channels and action potentials.
The primary role of the Na+-K+ pump (NKA) is to remove from the intracellular space the Na+ that enters during the action potential (36). The pump exchanges 3 Na+ for 2 K+ and thereby carries an outward current . It plays an important role in ionic homeostasis and also in cardiac repolarization.
The Na+-K+ pump needs energy to work, since it pumps out Na+ against its electrochemical gradient. The source of this energy comes from intracellular ATP, through the function of the Na+-K+-ATPase, which is part of the pump.
The main function of the Na+/Ca2+ exchanger (NCX) is to control calcium flux through the plasma membrane, and it transports 3 Na+ for 1 Ca2+, using the driving force of the Na+ gradient provided by the NKA.
There are several studies that link low magnesium to arrythmias or AF specifically:
Conclusions: "Low serum magnesium is moderately associated with the development of AF in individuals without cardiovascular disease. Because hypomagnesemia is common in the general population, a link with AF may have potential clinical implications. Further studies are warranted to confirm our findings and to elucidate the underlying mechanisms."
Conclusions: "Magnesium deficiency resulting from feeding a diet that would not be considered having an atypical menu induces heart arrhythmias ... in post menopausal women. A dietary intake of about 4.12 mmol (100 mg) Mg/8.4 MJ is inadequate for healthy adults and may result in compromised cardiovascular health ..."
Conclusion: "Lower serum Mg was associated with a higher AF risk, and this association was not different between whites and African Americans. Dietary Mg was not associated with AF risk."
Conclusion: "Further trial data may shed light on whether there is any role for magnesium in improving the management of patients with AF. However at present, the available data would suggest that magnesium, as an adjunct to electric cardioversion or for prevention, is more myth than a practical, easy (or magical) solution to the growing problem of AF."
Conclusions: "Magnesium has a number of potential beneficial effects on the cardiovascular system, most notably antiarrhythmic properties. This includes control of intracellular ion transport pumps responsible for movement of sodium (Na+), calcium (Ca2+), and potassium (K+) as well as reductions in EADs and slowed AV nodal conduction times. These physiologic properties provide promise of the therapeutic benefits that magnesium may have in managing various tachyarrhythmias. These benefits may stem from correcting the intracellular magnesium deficiency that has been found in many patient populations.
Taken together, a number of important clinical questions remain unanswered by this evidence base. The relationship between normalization of intracellular magnesium concentrations and improvements in clinical outcomes remains unknown. This includes pharmacologic investigations such as thorough QTc studies, correlations between magnesium levels and both surrogate and clinical outcomes, and dose-ranging studies. The most appropriate route (i.v. vs. oral), salt (oxide vs. lactate, etc.), dose, and duration of therapy for magnesium supplementation are also not available to clinicians. These gaps in evidence make incorporating the potentially important research findings into practice a challenge for clinicians caring for high-risk patients. They also represent critical need of study to allow the large body of evidence with magnesium to be translated to clinical practice."
"Recent data confirm a degree of interdependence between hypomagnesemia and cardiac arrhythmias."
"Changes in the surface electrocardiograms (ECG) associated with hypomagnesemia have been described over time, dependent and varying according to magnesium levels: ST segment depression, peaked and tall or flattened T waves, shortening of the PR interval and QTc, lowering of the QRS voltage, increasing in the QRS duration and the presence of U waves."
"Concerning the relationship between hypomagnesaemia and the development of atrial fibrillation in patients with no baseline cardiovascular disease, a large sample size and long follow-up study (20 years) showed that subjects with lower serum magnesium (≤1.77 mg/dL) had an approximately 50% increased risk of atrial fibrillation compared to those with magnesium levels in the upper limit."
"In a large study, mild and moderate hypomagnesemia was associated with a significantly increased incidence of atrial fibrillation over a 20-year follow-up"
"a recent two-phased randomised study on the effect of magnesium supplementation for PAC and PVC showed that even if magnesium is not a curative treatment for arrhythmia, it was associated with a reduction in premature complexes and symptoms in both phases"
Conclusions: "Magnesium is involved in multiple metabolic, enzymatic, and electrophysiological reactions and mechanisms which are indispensable or have additional benefits on the electrical stability of the ventricular myocardium. Although some studies reveal the association of hypomagnesemia with supraventricular and ventricular arrhythmias, this remains controversial and requires further research." "The benefit of magnesium for the reduction of cardiac arrhythmias seems to rely on several mechanisms: the regulation of intracellular ionic pumps for K+, Na+ and Ca2+, the reduction of early delayed after-depolarization triggering life-threatening ventricular arrhythmias, the decreasing of PAF- related inflammatory cardiac response linked to arrhythmias, the prevention of shortening of the action potential duration, the increasing of the atrioventricular node conduction time and the atrial and ventricular refractoriness. Magnesium supplementation in cardiac arrhythmias constitutes an interesting and stringent direction to investigate in order to translate knowledge into standardized and effective practical clinical directions."
Extracts from Magnesium: Biochemistry, Nutrition, Detection, and Social Impact of Diseases Linked to Its Deficiency. Florentini et al. 2021:
"Over 600 enzymes with Mg2+ as a cofactor are currently listed by the enzymatic databases, while an additional 200 are listed in which Mg2+ may act as an activator [38,39]. More specifically, it mainly interacts directly with the substrate, rather than acting as a real cofactor."
"Mg2+ acts as a cofactor in all reactions involving the utilization and transfer of ATP"
"Mg2+ participates in controlling the activity of some ionic channels in many tissues. Its mechanism of action relies on either direct interaction with the channel, or an indirect modification of channel function through other proteins (e.g., enzymes or G proteins), or via membrane surface charges and phospholipids [47]. Furthermore, Mg2+ acts as a physiological Ca2+ antagonist within cells, since it can compete with Ca2+ for binding sites in proteins and Ca2+ transporters [48]. These abilities are involved in the observed effect of magnesium on the cardiovascular system, muscle, and brain;"
"the Mg2+/Ca2+ ratio is very important for the activity of Ca2+-ATPases and other Ca2+ transporting proteins [48], thus small changes in the Mg2+ availability within the cell may cause perturbed Ca2+ signaling"
"Magnesium plays an important role in the cardiovascular system, influencing myocardial metabolism, Ca2+ homeostasis, and endothelium-dependent vasodilation. It also acts as an antihypertensive, antidysrhythmic, anti-inflammatory, and anticoagulant agent. In myocardium, the opening of L-type Ca2+ channels produces a long-lasting Ca2+ current, corresponding to the second phase of the cardiac action potential. Mg2+ inhibits these channels, preventing Ca2+ overload and cell toxicity and thus exerting a myocardial protective effect [98]. Two general mechanisms could explain how Mg2+ regulates Ca2+ fluxes through L-type channels: alteration of ion permeation and/or modulation of channel gating properties. L-type Ca2+ channel gating is, in turn, regulated by membrane potential, cytosolic Ca2+ concentration, and channel phosphorylation. It has been demonstrated that the effect of Mg2+ is dependent on the channel’s phosphorylation state, since phosphatase treatment decreases the inhibitory effect of Mg2+ [99]. Furthermore, Mg2+ is necessary for Na+/K+-ATPase, which is responsible for the active transport of K+ intracellularly during the action potential duration. Mg2+ is also involved in regulating the K+ influx through different K+ channels [100]. The modulation of cardiac action potential can explain the antidysrhythmic action of Mg2+: Its infusion provokes the slowing of atrioventricular nodal conduction, and also determines the prolongation of PR interval and QRS duration in the electrocardiogram [101]. The effect of Mg2+ on cardiomyocytes also depends on other mechanisms, including the ability of Mg2+ to compete with Ca2+ for binding sites in proteins, such as calmodulin, troponin C, and parvalbumin [48], to act as substrate in a complex with ATP for cardiac Ca2+-ATPases, and to alter the affinity of Na+-Ca2+ exchanger [2]. In summary, tight regulation of Mg2+ concentration in myocytes is necessary for optimal cardiac function, indeed hypomagnesemia can impact physiological activity, leading to cardiovascular diseases"
"Magnesium deficiency reduces cardiac Na-K-ATPase, determining greater levels of sodium and calcium and lower levels of magnesium and potassium in the heart. Consequently, the vasoconstriction in the coronary arteries increases, inducing coronary artery spasms, heart attack, and cardiac arrhythmia."
Extracts from Magnesium: Biochemistry, Nutrition, Detection, and Social Impact of Diseases Linked to Its Deficiency. Florentini et al. 2021:
"Older people absorb less magnesium from the gut and lose more magnesium because of an increased renal excretion. Chronic magnesium deficiency is indeed common in the elderly, usually due to a decrease both in diet assumption and intestinal absorption, and it is probably exacerbated by estrogen deficit, which occurs in aging women and men and cause hypermagnesuria"
"most apparently healthy people risk an insufficient magnesium intake due to a decreased presence of this metal in the modern Western diet characterized by a wide use of demineralized water, processed foods, and agricultural practices that use soil deficient in magnesium for growing food"
"data on people’s dietary habits still reveal that intakes of magnesium are lower than the recommended amounts either in the United States or in Europe. Epidemiological studies have shown that people consuming Western-type diets introduce an insufficient amount of micronutrients and in particular, a quantity of magnesium that is <30–50% of the RDA. Accordingly, the magnesium dietary intakes in the United States have been decreasing over the last 100 years from about 500 mg/day to 175–225 mg/day [21], and a general similar decrease in magnesium daily uptake in people fed a Western diet is reported"
Extracts from Subclinical magnesium deficiency: a principal driver of cardiovascular disease and a public health crisis. DiNicolantonio et al. 2018:
"There are two types of nutrient deficiencies, frank deficiencies (such as scurvy from ascorbic acid deficiency or goitre from iodine deficiency) and subclinical deficiencies (a clinically silent reduction in physiological, cellular and/or biochemical functions). It is the latter that is most concerning as it is hard to diagnose and predisposes to numerous chronic diseases. And while both result in negative health consequences, the former has obvious symptoms (hence frank deficiency), whereas the latter may have negative or variable health effects that are not so apparent (eg, vascular calcification). The evidence in the literature suggests that subclinical magnesium deficiency is rampant and one of the leading causes of chronic diseases including cardiovascular disease and early mortality around the globe, and should be considered a public health crisis."
"most people need an additional 300 mg of magnesium per day in order to lower their risk of developing numerous chronic diseases. So while the recommended daily allowance (RDA) for magnesium (between 300 and 420 mg/day for most people) may prevent frank magnesium deficiency, it is unlikely to provide optimal health and longevity, which should be the ultimate goal."
"Importantly, much of the population may not even be meeting the RDA for magnesium."
"The data are consistent around the world that magnesium intake may be inadequate."
"Hypomagnesemia is a relatively common occurrence in clinical medicine. That it often goes unrecognized is due to the fact that magnesium levels are rarely evaluated since few clinicians are aware of the many clinical states in which deficiency, or excess, of this ion may occur."
"Magnesium deficiency has been found in 84% of postmenopausal women with osteoporosis diagnosed by low magnesium trabecular bone content and Thoren’s magnesium load test."
"Magnesium deficiency can be present despite normal serum magnesium levels. ... our normal range of serum magnesium is inaccurate and that serum magnesium levels at the lower end of normal likely suggest marginal magnesium deficiency.29 Indeed, ‘The magnesium content of the plasma is an unreliable guide to body stores: muscle is a more accurate guide to the body content of this intracellular cation’. ... The muscle biopsy method is rapid, reliable and may reveal conditions of deficiency…oral supplements of Mg have proved to be adequate to restore the normal K/Mg status."
Extracts from Magnesium: Biochemistry, Nutrition, Detection, and Social Impact of Diseases Linked to Its Deficiency. Florentini et al. 2021:
"It is used to say that approximately 300 mg are ingested daily in the diet, however there are several factors that hinder or facilitate magnesium availability. Unfortunately, the bioavailability studies present in the literature cover a wide range of Mg2+ loading administration (i.e., from <100 to >1000 mg/day) and observed different periods of time. Moreover, other important variables, such as the age of subjects (infants—adults), their physical condition, and the proximity of magnesium administration to meals and different meal matrices, did not allow for a comparison of results, leading to confusing and apparently conflicting results. Obviously, systematic studies comparing Mg2+ absorption efficiency between magnesium-depleted and -saturated subjects were not possible due to ethical reasons."
"Approximately 30% to 40% of dietary magnesium consumption is usually absorbed by the body."
"In general, foods containing dietary non-fermentable fiber have indeed a high content of magnesium, nevertheless the bioavailability is low, analogously to iron.By contrast, fermentable low- or indigestible carbohydrates (e.g., inulin, oligosaccharides, resistant starch, mannitol, and lactulose) enhance Mg2+ uptake."
"Phytates and oxalates present in foods rich in fiber can decrease the absorption of magnesium because of metal chelation. Nevertheless, the decrease of magnesium absorption caused by phytate and cellulose is usually compensated by an increased magnesium intake due to high magnesium concentrations in phytate- and cellulose-rich products"
"Very high calcium intakes can reduce the absorption of magnesium, in particular, magnesium bioavailability decreases when calcium intake is over 10 mg/kg/day [18]. Increasing evidence suggests that the optimal serum magnesium/calcium ratio is 0.4 and if it is in the range 0.36–0.28, it is considered too low. This ratio is a more practical and sensitive of magnesium status and/or turnover, than the serum magnesium level alone"
"Dietary aluminum may contribute to a magnesium deficit by means of an approximately 5-fold reduction of its absorption, of 41% of its retention, and by causing a reduction of magnesium in the bone. Since aluminum is widespread in modern day society (such as in cookware, deodorants, over the counter and prescription drugs, powder, baked products, and others), this could represent an important contributor to magnesium deficiency"
"Vitamin D seems to have a favorable role on Mg2+ absorption and Mg2+ is important for vitamin D activation and inactivation"
"Vitamin B6 collaborates with magnesium in many enzyme systems and increases the accumulation of intracellular magnesium; a vitamin B6-deficient diet can lead to a negative magnesium balance via increased magnesium excretion"
"Magnesium deficiency can cause hypocalcemia [124]; Durlach suggested that the optimal dietary calcium:magnesium ratio is close to 2:1."
Extracts from Subclinical magnesium deficiency: a principal driver of cardiovascular disease and a public health crisis. DiNicolantonio et al. 2018:
"Supplementing with calcium can lead to magnesium deficiency due to competitive inhibition for absorption,84 and oversupplementing with vitamin D may lead to magnesium deficiency via excessive calcium absorption and hence increase the risk of arterial calcifications."
Extracts from Magnesium: Biochemistry, Nutrition, Detection, and Social Impact of Diseases Linked to Its Deficiency. Florentini et al. 2021:
"Magnesium is considered widely distributed in foods, although the amount of magnesium contained in food is influenced by various factors including the soil and water used to irrigate, fertilizers, conservation, and also refining, processing, and cooking methods. In general, seeds, legumes, nuts (almonds, cashews, Brazil nuts, and peanuts), whole grain breads, and cereals (brown rice, millet), some fruits, and cocoa are considered good sources of magnesium. Nevertheless, acidic, light, and sandy soil is usually deficient in magnesium content."
"Green leafy vegetables are frequently counted among the food rich in magnesium according to the hypothesis that chlorophyll-bound magnesium may represent important nutritional sources of magnesium. This hypothesis relies on what is known about iron ... This concept is incorrect for many reasons ... In leafy green vegetables, such as lettuce and spinach, chlorophyll-bound magnesium represents 2.5% to 10.5% of total magnesium, whereas other common green vegetables, pulses, and fruits contain <1% chlorophyll-bound magnesium. ... chlorophyll bound magnesium contributes a small and nutritionally insignificant part of total magnesium intake in industrialized countries"
" the nutrient content in foods must be critically analyzed because nutrient bioavailability and the amount of nutrients in food portions should also be taken into consideration"
The following table is from Florentini et al, but I have re-ordered the rows to be in descending USDA order:
| Food | EFSA (mg/100 g) | CREA (mg/100 g) | USDA (mg/Measure) | Measure and Weight |
|---|---|---|---|---|
| Pumpkin and squash seed, dried | 429 | 592 | 764 | 1 cup, 46 g |
| Amaranth flour | 266 | 266 | 476 | 1 cup, 193 g |
| Sweet, dried almonds | 251 | 264 | 386 | 1 cup, 143 g |
| Wheat/Cereal bran | 451 | 550 | 354 | 1 cup, 50 g |
| Cashews dried | 258 | 260 | 352 | 1 cup, 137 g |
| Quinoa | n.a | 189 | 335 | 1 cup, 170 g |
| Buckwheat flour, whole-groats | 121 | 231 | 301 | 1 cup, 120 g |
| Wheat germ | 276 | 255 | 275 | 1 cup, 115 g |
| Peanuts, roasted | 229 | 175 | 260 | 1 cup, 146 g |
| Beans, dried | 158 | 170 | 258 | 1 cup, 184 g |
| Millet, shelled | 136 | 160 | 228 | 1 cup, 200 g |
| Hazelnuts, dried | 163 | 163 | 187 | 1 cup, 187 g |
| Walnuts, dried | 150 | 158 | 185 | 1 cup, 169 g |
| Sunflower seeds dried | 346 | n.a 2 | 173 | 1 cup, 130 g |
| Wheat flour, hard | 136 | 120 | 164 | 1 cup, 120 g |
| Chickpeas, dried | 150 | 131 | 158 | 1 cup, 100 g |
| Macadamia | 115 | 120 | 156 | 1 cup, 132 g |
| Oat flour | 131 | n.a 2 | 150 | 1 cup, 169 g |
| Pistachios, dried | 147 | 160 | 149 | 1 cup, 123 g |
| Pecans | 168 | 121 | 132 | 1 cup, 109 g |
| Lentils, dried | 101 | 83.1 | 113 | 1 cup, 100 g |
| Wholemeal pasta | 111 | 101 | 95 | 1 cup, 90 g |
| Cocoa powder | 545 | 499 | 29 | 1 ts 1, 6 g |
"Pseudo cereal and whole-grain wheat, oat, and millet were shown to be great sources of magnesium even if the cooking methods influence the real magnesium assumption per portion. For example, 100 g of wholemeal pasta cooked in water contain 42 mg of magnesium. The introduction of unrefined whole grains, nuts, legumes, and unrefined dark chocolate in the daily diet is useful to reaching a satisfactory amount of magnesium, because they represent good dietary sources of magnesium [18]. Among fruit, a high content of magnesium is found in dried apricot and dried bananas even if the normal serving of dried fruit (30 g) contains a similar amount of magnesium to a serving (100–150 g) of some fresh fruit (e.g., avocado, blackberries, prickly pears, chokecherries)"
"Magnesium content in cocoa is at significant levels (2–4 mg/g dry powder). Therefore, a 40 g portion of 70–80%-cocoa dark chocolate would contain ≈40 mg of magnesium, enough to satisfy about ∼10% of the recommended daily allowance (300–400 mg magnesium/day in adults)"
Extracts from Subclinical magnesium deficiency: a principal driver of cardiovascular disease and a public health crisis. DiNicolantonio et al. 2018:
"Good dietary sources of magnesium include nuts, dark chocolate and unrefined whole grains."
"A common misconception is that consuming phytate-rich foods can lead to nutrient deficiencies particularly magnesium depletion via binding by phytic acid. However, urinary magnesium excretion will drop to compensate for a reduction in bioavailable magnesium.70 And most high-phytate foods are also good sources of magnesium (grains and beans are good examples). Thus, it is unlikely that consuming foods high in phytate will lead to magnesium depletion. However, a vitamin B6-deficient diet can lead to a negative magnesium balance via increased magnesium excretion."
Extracts from Magnesium: Biochemistry, Nutrition, Detection, and Social Impact of Diseases Linked to Its Deficiency. Florentini et al. 2021:
"Magnesium supplements are available in a variety of formulations, including inorganic salt (e.g., magnesium oxide, chloride, sulfate) and organic compounds (e.g., citrate, malate, pidolate, taurate). Magnesium absorption from different kinds of supplements is not the same, nevertheless, the results obtained in the available studies in humans are hardly comparable due to the differences among the study designs."
"In a recent review, Schuchardt and Hahn reported that the relative Mg2+ bioavailability is higher if the mineral is ingested in multiple low doses during the day rather than a single assumption of a high amount of Mg2+ [4]. Small studies showed that magnesium in the aspartate, chloride, citrate, and lactate salt is absorbed almost completely and is more bioavailable than magnesium oxide and magnesium sulfate [204,206]. In general, it has been suggested that absorption of organic magnesium salts is better than the absorption of inorganic compounds, whereas other studies did not find differences between salt formulations [4,200,207,208]. Unabsorbed magnesium salts in general cause diarrhea and laxative effects due to the osmotic activity in the intestine and colon and the stimulation of gastric motility."
"A daily supplement of 200 mg of chelated magnesium (citrate, lactate) is suggested to be likely safe, adequate, and sufficient to significantly increase serum magnesium concentration in a fasting non-hemolyzed serum sample to levels >0.85 mmol/L but <1.1 mmol/L. A steady state is usually achieved in 20–40 weeks of supplementation and is dependent on the dose."
"The beneficial effects of magnesium supplementation appeared to be more pronounced in the elderly and alcoholics, but were not particularly apparent in athletes and physically active individuals [217]. Further research on long-term administration of different magnesium compounds and their effect on other tissues are needed."
See NHS treatments for atrial fibrillation and NICE CKS prescribing information for atrial fibrillation.
Drug classes for treatment of atrial fibrillation: Class IA, IC, II, III, IV (non-DHP); digoxin
I haven't included rare or very rare side effects in these notes.
| Very common | ≥ 1/10 | ≥ 10% |
| Common | ≥ 1/100 and < 1/10 | ≥ 1% and < 10% |
| Uncommon | ≥ 1/1000 and < 1/100 | ≥ 0.1% and < 1% |
| Rare | ≥ 1/10,000 and < 1/1000 | ≥ 0.01% and < 0.1% |
| Very rare | < 1/10,000 | < 0.01% |
These notes only deal with NOAC/DOAC anticoagulants. I'm assuming that warfarin isn't relevant.
| BNF | Common or very common: Anaemia; haemorrhage; nausea; skin reactions Uncommon: CNS haemorrhage; hypotension; post procedural haematoma; thrombocytopenia; wound complications |
| NICE | Common: bleeding; anaemia, bruising, nausea, and skin reactions Uncommon: angioedema, erythema multiforme, hypotension, post procedural haematoma, thrombocytopenia, and wound complications |
| SmPC | Common: Anaemia; Eye haemorrhage (including conjunctival haemorrhage); Haemorrhage, haematoma; Hypotension (including procedural hypotension); Epistaxis; Nausea; Gastrointestinal haemorrhage; Rectal haemorrhage, gingival bleeding; Gamma-glutamyltransferase increased; Haematuria; Contusion |
| BNF | Common or very common: Anaemia; diarrhoea; gastrointestinal discomfort; haemorrhage; hepatic function abnormal; nausea Uncommon: Dysphagia; gastrointestinal disorders; hyperbilirubinaemia; intracranial haemorrhage; post procedural complications; skin reactions; thrombocytopenia; vomiting; wound complications |
| NICE | Common: bleeding; abnormal hepatic function |
| SmPC | Common: Anaemia; Epistaxis; Gastrointestinal haemorrhage; Abdominal pain; Diarrhoea; Dyspepsia; Nausea; Skin haemorrhage; Genitourological haemorrhage, including haematuria; |
| BNF | Common or very common: Abdominal pain; anaemia; dizziness; haemorrhage; headache; nausea; skin reactions Uncommon: CNS haemorrhage; thrombocytopenia |
| NICE | Common: bleeding; anaemia, dizziness, gastrointestinal disorders, headache, pruritus, rash, nausea, and abnormal liver function tests Uncommon: thrombocytopenia, hypersensitivity, and urticaria |
| SmPC | Common: Anaemia; Dizziness; Headache; Epistaxis; Abdominal pain; Lower GI haemorrhage; Upper GI haemorrhage; Oral/pharyngeal haemorrhage; Nausea; Blood bilirubin increased; Gammaglutamyltransferase increased; Cutaneous soft tissue haemorrhage; Rash; Pruritus; Macroscopic haematuria/urethral haemorrhage; Vaginal haemorrhage; Puncture site haemorrhage; Liver function test abnormal |
| BNF | Common or very common: Anaemia; asthenia; constipation; diarrhoea; dizziness; fever; gastrointestinal discomfort; haemorrhage; headache; hypotension; menorrhagia; nausea; oedema; pain in extremity; post procedural anaemia; renal impairment; skin reactions; vomiting; wound complications Uncommon: Angioedema; dry mouth; hepatic disorders; hypersensitivity; intracranial haemorrhage; malaise; syncope; tachycardia; thrombocytopenia; thrombocytosis |
| NICE | Common: bleeding; anaemia, fatigue, asthenia, constipation, diarrhoea, dizziness, fever, gastrointestinal discomfort, dyspepsia, constipation, headache, hypotension, haematoma, menorrhagia, nausea, vomiting, oedema, pain in extremity, pruritus, rash, and peripheral oedema Uncommon: angioedema and allergic oedema, allergic reaction, allergic dermatitis, dry mouth, feeling unwell, hepatic disorders, haemarthrosis, hypersensitivity, malaise, syncope, tachycardia, thrombocytopenia, thrombocytosis, and urticaria |
| SmPC | Common: Anaemia; Dizziness, headache; Eye haemorrhage; Hypotension, haematoma; Epistaxis, haemoptysis; Gingival bleeding, gastrointestinal tract haemorrhage; Increase in transaminases; Pruritus; Pain in extremity; Urogenital tract haemorrhage; Fever, peripheral oedema, decreased general strength and energy; Postprocedural haemorrhage |
Table from CV Physiology:
| Class IC: life-threatening supraventricular tachyarrhythmias (SVT) and ventricular tachyarrhythmias (VT) | ||
| flecainide* | SVT | can induce life-threatening VT |
| propafenone | SVT & VT; | β-blocking and Ca++-channel blocking activity can worsen heart failure |
| BNF | |
| CV Physiology |
| BNF | Supraventricular arrhythmias (initiated under direction of hospital consultant) |
| CV Physiology | life-threatening supraventricular tachyarrhythmias (SVT) and ventricular tachyarrhythmias (VT) |
| BNF | |
| CV Physiology | IC compounds can cause increased risk of sudden death in patients with a prior history of myocardial infarction or sustained ventricular arrhythmias. |
| BNF | elderly (accumulation may occur) |
| CV Physiology |
| BNF | Fludrocortisone is predicted to cause hypokalaemia (potentially increasing the risk of torsade de pointes) when given with Flecainide. Severity:Severe |
| NICE |
Beta blockers: The combination of a beta-blocker and a class I anti-arrhythmic is not recommended because bradycardia and myocardial depression can occur. |
| BNF | Common: Arrhythmias; asthenia;
dizziness; dyspnoea; fever; oedema; vision
disorders Uncommon: Alopecia; appetite decreased; constipation; diarrhoea; flatulence; gastrointestinal discomfort; nausea; skin reactions; vomiting |
| CV Physiology | |
| SmPC |
Very common: dizziness, which is
usually transient; visual impairment, such as
diplopia and vision blurred |
| BNF | |
| CV Physiology |
| BNF | Ventricular arrhythmias (specialist supervision in
hospital) Paroxysmal supraventricular tachyarrhythmias which include paroxysmal atrial flutter or fibrillation and paroxysmal re-entrant tachycardias involving the AV node or accessory pathway, where standard therapy ineffective or contra-indicated (specialist supervision in hospital) |
| CV Physiology | life-threatening supraventricular tachyarrhythmias (SVT) and ventricular tachyarrhythmias (VT) |
| NICE | Beta blockers: The combination of a beta-blocker and a class I anti-arrhythmic is not recommended because bradycardia and myocardial depression can occur. |
| BNF | marked hypotension; severe bradycardia |
| CV Physiology | IC compounds can cause increased risk of sudden death in patients with a prior history of myocardial infarction or sustained ventricular arrhythmias. |
| BNF | Elderly |
| CV Physiology |
| BNF | |
| CV Physiology |
| BNF | Common or very common: Anxiety;
arrhythmias; asthenia; cardiac conduction disorder;
chest pain; constipation; diarrhoea; dizziness; dry
mouth; dyspnoea; fever; gastrointestinal discomfort;
headache; hepatic disorders; nausea; palpitations;
sleep disorders; taste altered; vision blurred;
vomiting Uncommon: Appetite decreased; erectile dysfunction; gastrointestinal disorders; hypotension; movement disorders; paraesthesia; skin reactions; syncope; thrombocytopenia; vertigo |
| CV Physiology | |
| SmPC | Very common: Dizziness, Cardiac
conduction disorders, Palpitations Common: Anxiety, Sleep disorders, Headache, Dysgeusia, Vision blurred, Sinus bradycardia, Bradycardia, Tachycardia, Atrial flutter, Dyspnoea, Abdominal pain, Vomiting, Nausea, Diarrhoea, Constipation, Dry mouth, Hepatic function abnormal, Chest pain, Asthenia, Fatigue, Pyrexia Uncommon: Thrombocytopenia, Decreased appetite, Nightmare, Syncope, Ataxia, Paraesthesia, Vertigo, Ventricular tachycardia, Arrythmia, Hypotension, Abdominal distension, Flatulence, Urticaria, Pruritus, Rash, Erythema, Erectile dysfunction |
Table from CV Physiology:
| Clinical Uses | ||||||
| Class/Drug | HTN | Angina | Arrhy | MI | CHF | Comments |
| Non-selective β1/β2 | ||||||
| nadolol | X | X | X | X | long acting | |
| propranolol | X | X | X | X | MSA; prototypical beta-blocker | |
| sotalol | X | several other significant mechanisms | ||||
| β1-selective | ||||||
| acebutolol | X | X | X | ISA; MSA | ||
| atenolol | X | X | X | X | ||
| bisoprolol | X | X | X | X | ||
| metoprolol | X | X | X | X | X | |
| BNF | Arrhythmias (FOG: except bisoprolol!) |
| CV Physiology | Arrhythmias |
NICE:
| BNF | hypotension; marked bradycardia |
| CV Physiology | |
| NICE | Sinus bradycardia (heart rate less than 60 beats per
minute at the start of treatment). Severe hypotension (systolic less than 100 mmHg). |
| BNF | Elderly: Prescription potentially inappropriate (STOPP criteria) with bradycardia (heart rate less than 50 beats per minute). |
| CV Physiology |
| BNF | Both beta-blocker and Alcohol can increase the risk of hypotension. |
| NICE | Corticosteroids, oestrogens — hypotensive effect
antagonised with concomitant use. (FOG: can't find any
evidence to support interaction with oestrogens). Class I anti-arrhythmics (such as quinidine, flecainide): The combination of a beta-blocker and a class I anti-arrhythmic is not recommended because bradycardia and myocardial depression can occur. |
| BNF | Common or very common: Abdominal discomfort;
bradycardia; confusion; depression; diarrhoea;
dizziness; dry eye (reversible on
discontinuation); dyspnoea; erectile dysfunction;
fatigue; headache; heart failure; nausea; paraesthesia;
peripheral coldness; peripheral vascular disease;
rash (reversible on discontinuation); sleep disorders;
syncope; visual impairment; vomiting Atrioventricular block; bronchospasm |
| CV Physiology | bradycardia |
| NICE | Bradycardia. Bronchospasm. Cold extremities, paraesthesiae, and numbness — these are more common in people with peripheral vascular disease. If troublesome, beta-blockers might need to be stopped. Conduction disorders. Dizziness. Dyspnoea. Exacerbation of psoriasis. Exacerbation of Raynaud's phenomenon. Fatigue — an incidence of approximately 18 per 1,000 people treated with a beta-blocker has been reported. Gastrointestinal disturbances. Headache. Heart failure. Hyperglycaemia/hypoglycaemia — in people with or without diabetes. Hypotension. Impotence and loss of libido — occurs in approximately five people per 1,000 receiving treatment. Paraesthesia. Peripheral vasoconstriction. Psychoses. Purpura. Sleep disturbance or nightmares — this occurs less frequently with water-soluble beta-blockers, such as atenolol, because these drugs are less likely to cross the blood-brain barrier. Thrombocytopenia. Vertigo. Visual disturbances |
See details above for all beta-blockers. This is additional information that is specific to this drug.
| BNF | Uncommon: Appetite decreased; behaviour abnormal; constipation; cough; dry mouth; dyspepsia; facial swelling; flatulence; hyperhidrosis; nasal congestion; sedation; sexual dysfunction; skin reactions; speech slurred; tinnitus; vision blurred; weight increased |
| CV Physiology | |
| SmPC | Common: Bradycardia (heart rate <60
BPM); Heart rate <40 BPM and or symptomatic
bradycardia (approx. 2%); Cardiac
failure; Rhythm/conduction disturbances (about
1%); Symptoms of peripheral vascular insufficiency
usually of the Raynaud type (approx. 2%); Dizziness
(approx. 2%); Fatigue (approx. 2%) Uncommon: Paraesthesias and sedation (approx. 0.6%); Headache and slurred speech (0.1 to 0.5%); Change in behaviour (approx. 2%); Nausea, diarrhoea, abdominal discomfort, constipation, vomiting, indigestion, bloating and flatulence (0.1-0.5%); Dry mouth (0.1 – 0.5%); Anorexia (0.1% to 0.5%); Cough and nasal stiffness (0.1% to 0.5%); Bronchospasm (approx. 0.1%) (see section 4.3 Contraindications and section 4.4 Special warnings and precautions for use); Rash, pruritus; dry skin; facial swelling and sweating 0.1% to 0.5%); Weight gain (0.1% to 0.5% of patients); Impotence or decreased libido (0.1% to 0.5%); Dry eyes and blurred vision (0.1% to 0.5%); Tinnitus (0.1% to 0.5%) |
See details above for all beta-blockers. This is additional information that is specific to this drug.
| SmPC | Oestrogen and progestrogens, as used in the contraceptive pill, when taken with propranolol may antagonise the hypotensive effect. |
| SmPC | Common: Fatigue and/or lassitude (often
transient); Bradycardia, cold extremities,
Raynaud's phenomenon; Sleep disturbances,
nightmares Uncommon: Gastrointestinal disturbance, such as nausea, vomiting, diarrhoea |
See details above for all beta-blockers. This is additional information that is specific to this drug.
| BNF | Common or very common: Gastrointestinal disorder |
| CV Physiology | |
| SmPC |
Very common: Antinuclear
antibody; Fatigue; Gastrointestinal
disorders |
See details above for all beta-blockers. This is additional information that is specific to this drug.
| BNF | Common or very common: Gastrointestinal disorder |
| CV Physiology | |
| SmPC |
Common: Bradycardia; Cold
extremities; Gastrointestinal
disturbances; Fatigue |
See details above for all beta-blockers. This is additional information that is specific to this drug.
| BNF | FOG: not indicated for arrythmias! |
| CV Physiology | Arrhythmias |
| BNF | Common or very common: Constipation Uncommon: Muscle cramps; muscle weakness; postural hypotension |
| CV Physiology | |
| SmPC | Very common: bradycardia Common: dizziness, headache; worsening of heart failure; feeling of coldness or numbness in the extremities, hypotension; gastrointestinal complaints such as nausea, vomiting, diarrhoea, constipation; asthenia, fatigue Uncommon: sleep disorders, depression; AV- conduction disturbances; orthostatic hypotension; bronchospasm in patients with bronchial asthma or a history of obstructive airways disease; muscular weakness and cramps |
See details above for all beta-blockers. This is additional information that is specific to this drug.
| BNF | Common or very common: Constipation; palpitations; postural disorders |
| CV Physiology | |
| SmPC | Very common: Pronounced blood pressure drop and
orthostatic hypotension, very rarely with
syncope; Fatigue Common: Dizziness, headache; Bradycardia, balance disturbances (very rarely with associated syncope), palpitations; Cold hands and feet; Functional dyspnea; Nausea, abdominal pain, diarrhoea, constipation Uncommon: Weight gain; Depression, concentration problems, drowsiness or insomnia, nightmares; Paresthesia; Temporary exacerbation of symptoms of heart failure, first-degree atrioventricular block, precordial pain; Bronchospasms; Vomiting; Rash (psoriasislike urticaria and dystrophic cutaneous lesions), increased perspiration; Muscle spasms; Oedema |
Table from CV Physiology:
| Drug | Therapeutic Uses | Comments |
| amiodarone | ventricular tachycardia, including ventricular fibrillation; atrial fibrillation and flutter (off-label use) | very long half-life (25-60 days); Class I, II, III & IV actions and therefore decreases phase 4 slope and conduction velocity; side effects include (some serious): pulmonary fibrosis; hypothyroidism; hepatotoxicity; corneal micro-deposits, skin discoloration |
| dronedarone | atrial fibrillation (non-permanent) and flutter | structurally related (but non-iodinated) to amiodarone, but has a much smaller volume of distribution and shorter elimination half-life (~24 hr); Class I, II, III & IV actions; contraindicated in severe or recently decompensated, symptomatic heart failure; although less toxic than amiodarone, it is less efficacious in atrial fibrillation |
| bretylium | life-threatening ventricular tachycardia and fibrillation | IV only; initial sympathomimetic effect (norepinephrine release) followed by inhibition, which can lead to hypotension |
| sotalol | ventricular tachycardia; atrial flutter and fibrillation | inhibits opening of repolarizing K+ channels and increases ERP; also has Class II (beta-blocker) activity and therefore slows sinus rate |
| ibutilide | atrial flutter and fibrillation (acute termination) | activates slow inward Na+ currents during early phase 3 and inhibits opening of repolarizing K+ channels, which delays repolarization and increases ERP; IV only; can cause life-threatening ventricular arrhythmias; infrequent non-cardiac side effects |
| dofetilide | atrial flutter and fibrillation; paroxysmal supraventricular tachycardia (off-label) | approved for acute atrial flutter and fibrillation; very selective K+-channel blocker; can cause life-threatening ventricular arrhythmias |
| BNF | |
| CV Physiology |
| BNF | Treatment of arrhythmias, particularly when other drugs are ineffective or contra-indicated (including paroxysmal supraventricular, nodal and ventricular tachycardias, atrial fibrillation and flutter, ventricular fibrillation, and tachyarrhythmias associated with Wolff-Parkinson-White syndrome) (initiated in hospital or under specialist supervision) |
| CV Physiology | ventricular tachycardia, including ventricular fibrillation; atrial fibrillation and flutter (off-label use) |
| BNF | sinus bradycardia (except in cardiac arrest) |
| CV Physiology |
| BNF | Healthcare professionals are reminded that amiodarone
can cause serious adverse reactions affecting the eyes, heart,
lung, liver, thyroid gland, skin, and peripheral nervous system
that may persist for a month or longer after treatment
discontinuation. Some of these reactions may be
life-threatening but onset can be delayed; patients should be
supervised and reviewed regularly, especially those on
long-term treatment. Liver and thyroid function tests should be
performed. Elderly: Prescription potentially inappropriate (STOPP criteria) as first-line antiarrhythmic therapy in supraventricular tachyarrhythmias (higher risk of side-effects than beta-blockers, digoxin, verapamil or diltiazem) |
| CV Physiology | very long half-life (25-60 days) |
| BNF | Fludrocortisone is predicted to cause hypokalaemia
(potentially increasing the risk of torsade de pointes) when
given with Amiodarone. Severity:Severe Amiodarone slightly increases the exposure to Edoxaban. Severity:Severe |
| CV Physiology | |
| NICE | The combination of a beta-blocker and amiodarone should be prescribed with caution as there is an increased risk of bradycardia, atrioventricular (AV) block, and myocardial depression — monitor pulse and blood pressure and check for signs of worsening heart failure |
| BNF | Common or very common: Arrhythmias; hepatic
disorders; hyperthyroidism; nausea; respiratory disorders; skin
reactions Rare or very rare: Bronchospasm (in patients with severe respiratory failure); headache; idiopathic intracranial hypertension; nerve disorders; SIADH |
| CV Physiology | pulmonary fibrosis; hypothyroidism; hepatotoxicity;
corneal micro-deposits, skin discoloration bradycardia and atrioventricular block |
| SmPC |
Very common: corneal microdeposits
usually limited to the area under the pupil, which are
usually only discernable by slit-lamp examinations. They may
be associated with coloured halos in dazzling light or
blurred vision. Corneal micro-deposits consist of complex
lipid deposits and are reversible following discontinuation
of treatment. The deposits are considered essentially benign
and do not require discontinuation of amiodarone; benign
gastrointestinal disorders (nausea, vomiting, dysgeusia)
usually occurring with loading dosage and resolving with dose
reduction; isolated increase in serum transaminases,
which is usually moderate (1.5 to 3 times normal range),
occurring at the beginning of therapy. It may return to
normal with dose reduction or even
spontaneously; photosensitivity |
| BNF | Dronedarone is a multi-channel blocking anti-arrhythmic drug. |
| CV Physiology |
| BNF | Maintenance of sinus rhythm after cardioversion in clinically stable patients with paroxysmal or persistent atrial fibrillation, when alternative treatments are unsuitable (initiated under specialist supervision) |
| CV Physiology | atrial fibrillation (non-permanent) and flutter although less toxic than amiodarone, it is less efficacious in atrial fibrillation |
| SmPC | maintenance of sinus rhythm after successful cardioversion in adult clinically stable patients with paroxysmal or persistent atrial fibrillation (AF). Due to its safety profile (see sections 4.3 and 4.4), Dronedarone should only be prescribed after alternative treatment options have been considered. |
| BNF | bradycardia |
| CV Physiology | |
| SmPC | Bradycardia <50 beats per minute (bpm) |
| BNF | Coronary artery disease; correct hypokalaemia and hypomagnesaemia before starting and during treatment |
| CV Physiology |
| BNF | Fludrocortisone is predicted to cause hypokalaemia
(potentially increasing the risk of torsade de pointes) when
given with Dronedarone. Severity:Severe Dronedarone slightly increases the exposure to Edoxaban. Manufacturer advises adjust Edoxaban dose. Severity:Severe |
| CV Physiology |
| BNF | Common or very common: Asthenia;
bradycardia; congestive heart failure; diarrhoea;
gastrointestinal discomfort; nausea; QT interval prolongation;
skin reactions; vomiting Uncommon: Photosensitivity reaction; respiratory disorders; taste altered |
| CV Physiology | bradycardia and atrioventricular block |
| SmPC |
Very common: Congestive heart
failure |
| BNF | |
| CV Physiology |
| BNF | Prophylaxis of paroxysmal atrial tachycardia or
fibrillation, paroxysmal AV re-entrant tachycardias (both nodal
and involving accessory pathways), and paroxysmal
supraventricular tachycardia after cardiac surgery, Maintenance of sinus rhythm following cardioversion of atrial fibrillation or flutter |
| CV Physiology | ventricular tachycardia; atrial flutter and fibrillation |
| BNF | hypotension; marked bradycardia |
| CV Physiology |
| BNF | Sotalol may prolong the QT interval, and it occasionally
causes life threatening ventricular arrhythmias (important:
manufacturer advises particular care is required to avoid
hypokalaemia in patients taking sotalol—electrolyte
disturbances, particularly hypokalaemia and hypomagnesaemia
should be corrected before sotalol started and during use). Elderly: Prescription potentially inappropriate (STOPP criteria) with bradycardia (heart rate less than 50 beats per minute) Measurement of corrected QT interval, and monitoring of ECG and electrolytes required; correct hypokalaemia, hypomagnesaemia, or other electrolyte disturbances. |
| CV Physiology |
| BNF | Fludrocortisone is predicted to cause hypokalaemia (potentially increasing the risk of torsade de pointes) when given with Sotalol. Severity:Severe |
| CV Physiology |
| BNF | Common: Abdominal discomfort;
bradycardia; confusion; depression; diarrhoea;
dizziness; dry eye (reversible on
discontinuation); dyspnoea; erectile dysfunction;
fatigue; headache; heart failure; nausea; paraesthesia;
peripheral coldness; peripheral vascular disease;
rash (reversible on discontinuation); sleep disorders;
syncope; visual impairment; vomiting. Common or very common: Anxiety; arrhythmia; chest pain; dyspepsia; fever; flatulence; hearing impairment; mood altered; muscle spasms; oedema; palpitations; sexual dysfunction; taste altered; torsade de pointes (increased risk in females) Uncommon: Atrioventricular block; bronchospasm |
| SmPC | Common: Bradycardia, dyspnoea,
chest pain, palpitations, oedema, ECG
abnormalities, hypotension, arrhythmia,
syncope, cardiac failure,
presyncope; Rash; Nausea, vomiting, diarrhoea,
dyspepsia, abdominal pain, flatulence; Muscle
spasms; Fatigue, dizziness, asthenia,
light-headedness, headache, paraesthesia,
dysgeusia; Sleep disorder, mood altered,
depression, anxiety; Sexual dysfunction; Visual
disturbances; Hearing disturbances; Pyrexia Uncommon: Alopecia, Hyperhidrosis; |
Non-dihydropyridines:
| BNF | Calcium-channel blockers (less correctly called ‘calcium-antagonists’) interfere with the inward displacement of calcium ions through the slow channels of active cell membranes. They influence the myocardial cells, the cells within the specialised conducting system of the heart, and the cells of vascular smooth muscle. Thus, myocardial contractility may be reduced, the formation and propagation of electrical impulses within the heart may be depressed, and coronary or systemic vascular tone may be diminished. |
| CV Physiology |
| BNF | |
| CV Physiology |
| BNF | Treatment of supraventricular arrhythmias Paroxysmal tachyarrhythmias |
| CV Physiology | arrhythmias |
| BNF | bradycardia; hypotension |
| CV Physiology | bradycardia |
| NICE | Severe bradycardia (below 50 beats per minute) Severe hypotension (systolic blood pressure less than 90 mmHg). |
| BNF | Elderly: Prescription potentially inappropriate (STOPP criteria) with persistent postural hypotension i.e. recurrent drop in systolic blood pressure ≥ 20 mmHg (risk of syncope and falls). |
| CV Physiology |
| BNF | Verapamil slightly increases the exposure to
Edoxaban. Severity:Severe Both Verapamil and Alcohol can increase the risk of hypotension. |
| CV Physiology | |
| NICE | The combination of a beta-blocker and verapamil should not be prescribed because bradycardia, asystole, severe hypotension, and heart failure can occur. |
| BNF | Common or very common: Abdominal pain;
dizziness; drowsiness; flushing; headache;
nausea; palpitations; peripheral oedema; skin
reactions; tachycardia; vomiting. Hypotension. Uncommon: Angioedema; depression; erectile dysfunction; gingival hyperplasia; myalgia; paraesthesia; syncope |
| CV Physiology | bradycardia, constipation, impaired electrical conduction (e.g., atrioventricular nodal block), and depressed cardiac contractility |
| NICE | Common: constipation, which may be improved by
eating more fibre and drinking plenty of fluids. Less commonly: nausea, vomiting, dizziness, and fatigue have also been reported. Bradycardia. |
SmPC
| BNF | |
| CV Physiology |
| BNF | Not indicated for arrythmias! |
| CV Physiology | |
| SmPC | Not indicated for arrythmias! |
| BNF | severe bradycardia |
| CV Physiology | bradycardia |
| NICE |
Severe bradycardia (below 40 beats per minute). |
| BNF | Bradycardia (avoid if severe) Elderly: Prescription potentially inappropriate (STOPP criteria) with persistent postural hypotension i.e. recurrent drop in systolic blood pressure ≥ 20 mmHg (risk of syncope and falls). |
| CV Physiology |
| BNF | Both Diltiazem and Alcohol can increase the risk of hypotension |
| CV Physiology | |
| NICE | Caution should be used if prescribing diltiazem with a beta-blocker — monitor pulse and blood pressure carefully, as bradycardia and atrioventricular block can occur. Asystole and sudden death have also been reported. |
| BNF | Common or very common: Abdominal pain; dizziness; drowsiness; flushing; headache; nausea; palpitations; peripheral oedema; skin reactions; tachycardia; vomiting |
| CV Physiology | bradycardia, constipation, impaired electrical conduction (e.g., atrioventricular nodal block), and depressed cardiac contractility. |
| NICE | Common: dizziness, atrioventricular block, palpitations, gastrointestinal disorders (for example constipation, nausea, and dyspepsia), erythema, malaise, and fatigue. Bradycardia. |
| SmPC | Very common: Peripheral oedema Common: Headache, dizziness; Atrioventricular block (may be of first, second or third degree; bundle branch block may occur), palpitations; Flushing; Constipation, dyspepsia, gastric pain, nausea; Erythema, pruritus; Malaise, fatigue |
| BNF | Digoxin is a cardiac glycoside that increases the force of myocardial contraction and reduces conductivity within the atrioventricular (AV) node. |
| CV Physiology | The mechanism of this beneficial effect of digoxin is its ability to activate vagal efferent nerves to the heart (parasympathomimetic effect). Vagal activation can reduce the conduction of electrical impulses within the atrioventricular node to the point where some of the impulses will be blocked. When this occurs, fewer impulses reach the ventricles and ventricular rate falls. Digoxin also increases the effective refractory period within the atrioventricular node. |
| BNF | Rapid digitalisation, for atrial fibrillation or flutter
(24 hours) Maintenance, for atrial fibrillation or flutter Emergency loading dose, for atrial fibrillation or flutter (intravenous, rarely needed) |
| CV Physiology | Atrial fibrillation and flutter |
| BNF | |
| CV Physiology |
| BNF | |
| CV Physiology | Elderly patients are more susceptible to digoxin
toxicity because they often have reduced renal
function, and their reduced muscle mass increases plasma
digoxin levels. A 2018 analysis of the AFFIRM trial determined that digoxin significantly increased all-cause mortality in patients with atrial fibrillation. This calls into question the practice of using digoxin for lowering ventricular rate in patients with atrial fibrillation. |
| SmPC | Arrhythmias may be precipitated by digoxin toxicity, some of which can resemble arrhythmias for which the drug could be advised. For example, atrial tachycardia with varying atrioventricular block requires particular care as clinically the rhythm resembles atrial fibrillation. |
| BNF | Fludrocortisone is predicted to increase the risk of Digoxin toxicity when given with Digoxin. Severity:Severe |
| CV Physiology | |
| NICE | Concomitant administration of a beta-blocker and digoxin can reduce heart rate and prolong AV conduction time, increasing the risk of AV block and bradycardia. Monitor the pulse carefully; consider monitoring with electrocardiography (ECG). |
| BNF | Common or very common: Arrhythmias; cardiac
conduction disorder; cerebral impairment; diarrhoea; dizziness;
eosinophilia; nausea; skin reactions; vision disorders;
vomiting Uncommon: Depression |
| CV Physiology | The major side effect of digoxin is cardiac arrhythmia, especially atrial tachycardias and atrioventricular block. |
| SmPC |
Common: Nervous system disorder,
dizziness; Visual impairment (blurred
vision or xanthopsia); Arrhythmia, conduction
disorder, bigeminy, trigeminy, PR prolongation, sinus
bradycardia; Nausea, vomiting,
diarrhoea; Rash; Gynaecomastia; Fatigue,
malaise, asthenia |
"Tachycardia" is defined, for adults, as a heart rate greater than 100 beats/minute (see, for example, Wikipedia), where "heart rate" is defined as the number of ventricular contractions per minute (see, for example, physio-pedia).
Tachycardia "may be normal (such as with exercise) or abnormal (such as with electrical problems within the heart)." (Wikipedia).
A resting heart rate greater than 100 is always considered to be abnormal.
"Tachyarrhythmia" is sometimes used for "abnormal tachycardia", and confusingly "tachycardia" is sometimes used to mean "abnormal tachycardia" (i.e. "tachyarrhythmia").
Tachycardia is classified as follows (see, for example, Wikipedia, heart.org, litfl or webmd):
There are various types of supraventricular tachycardia (SVT), but usage of terminology seems to vary.
"Technically atrial fibrillation, atrial flutter and even physiological (e.g. exercise induced) tachycardia fall under SVT. In clinical practice, however, SVT usually refers to AV node re-entry tachycardia (AVNRT), whereby, a re-entry circuit forms within or near the AV node. Other examples of SVT include atrioventricular re-entry tachycardia (AVRT) where an accessory pathway forms, such as the Bundle of Kent, that is not part of or near the AV node, or junctional tachycardias" (NHS Scotland).
"Atrial tachycardia" is sometimes used as a synonym for SVT (e.g. heart.org).
"Supraventricular tachycardia (SVT) is a heterogeneous group of arrhythmias used to describe tachycardias that involve cardiac tissue at the level of the bundle of His or above." (Supraventricular tachycardia: An overview of diagnosis and management).
Note that the AV-node is above the bundle of His and so can be an origin of SVT.
Atrial fibrillation seems to be the only form of SVT in which the atria do not contract. The P wave will be retrograde (hence inverted in lead II) if the origin is the AV-node or low down in the atria.
Life in the Fast Lane have the most systematic classification and the best descriptions that I have found (links below are to Life in the Fast Lane descriptions):
Definitions from Collins dictionary.
| Embolism | A serious medical condition that occurs when an artery becomes blocked, usually by a blood clot. |
| Embolus | Material, such as part of a blood clot or an air bubble, that is transported by the bloodstream until it becomes lodged within a small vessel and impedes the circulation. |
| Fibrin | A white insoluble elastic protein formed from fibrinogen when blood clots: forms a network that traps red cells and platelets. |
| Fibrinolysis | The breakdown of fibrin in blood clots, esp by enzymes. |
| Ischaemia | An inadequate supply of blood to an organ or part, as from an obstructed blood flow. |
| Plasmin | A proteolytic enzyme that causes fibrinolysis in blood clots. |
| Platelet | Platelets are a kind of blood cell. If you cut yourself and you are bleeding, platelets help to stop the bleeding. |
| Thrombin | An enzyme that acts on fibrinogen in blood causing it to clot. |
| Thrombosis | The formation of a blood clot in a person's heart or in one of their blood vessels, which can cause death. |
| Thrombus | A clot of coagulated blood that forms within a blood vessel or inside the heart and remains at the site of its formation, often impeding the flow of blood. |